1. Energy is the ability to do work. Work is the transfer of energy from one body to another. Metabolism is the sum of all anabolic and catabolic process in a cell or organism
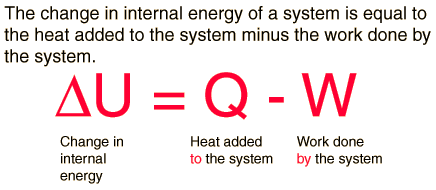
2. First law of thermodynamic – the total amount of energy in the universe is constant. Energy cannot be created or destroyed but only converted from one form into another.
3. Kinetic energy is the energy possessed by moving object. Potential energy is the energy stored by virtue of an object’s position within an attractive or repulsive force field.
4. Bond energy is the minimum energy required to break one mole of bond between two species of atom.
5. To show the change in potential energy that take place during a chemical reaction one would need a potential energy diagram.
6. Activation energy is the difference between the potential energy level of the transition state and the potential energy of reacting molecules.
7. Transition state in a chemical reaction is a temporary condition in which bonds within reactants are breaking and the bonds between products are forming.
8. Exothermic reaction results in a net amount of energy released, while endothermic reaction results in a net amount of energy absorbed. An example of exothermic reaction is combustion.
9. Entropy is a measure of the randomness or disorder in energy or in a collection of object.
10. Exothermic reactions that increase in entropy occur spontaneously at all temperature. If it decreases in entropy, the reaction only occurs spontaneously at low temperature. Endothermic reaction that increases entropy only occurs spontaneously in high temperature and if it doesn’t increase entropy it will only occur with a net input of energy.
11. Free energy is energy that can do useful work.
12. The second law of thermodynamics – the entropy of the universe increases with any change that occurs.
13. Exergonic reaction is a chemical reaction in which the energy of the product is less than the energy if the reactant. This is related to exothermic reaction. Endergonic reaction is a chemical reaction in which the energy of the product is more that the energy of the reactant. This is related to Endothermic reaction
14. Metabolic reactions are reversible. When reversible reaction reaches equilibrium they become a dead cell.
16. Redox reaction is a chemical reaction involving oxidation (atom loses one or more electron) and reduction (gaining one or more electron). The substance that loses an electron is called reducing agent while the substance that gains an electron is called an oxidizing agent.
17. Enzymes are used to catalyze a reaction. Substrates are the reactant that an enzyme acts on when it catalyzes chemical reaction. Active site is the location where the substrate binds to an enzyme. The interaction between the substrate and the amino acids causes the protein to change its shape. This is known as the induced-fit model.
18. Enzyme requires cofactor or coenzymes to work properly.
19. Enzyme inhibitors are used to control enzyme activity. Enzyme activities can also be controlled by allosteric regulation. Feedback inhibition is a method of metabolic control in which product formed later in a sequence of reaction allosterically inhibits an enzyme that catalyzes a reaction occurring earlier in the process.
20. There are many commercial and industrial use of enzyme such as using proteases to coagulate milk for the manufacture of cheese.



No comments:
Post a Comment